
Moving Our Pipeline Forward — We’re Pioneering New Possibilities With ONS-5010
Our development pipeline includes ongoing research of the first ophthalmic formulation of bevacizumab (ONS-5010) across multiple indications.
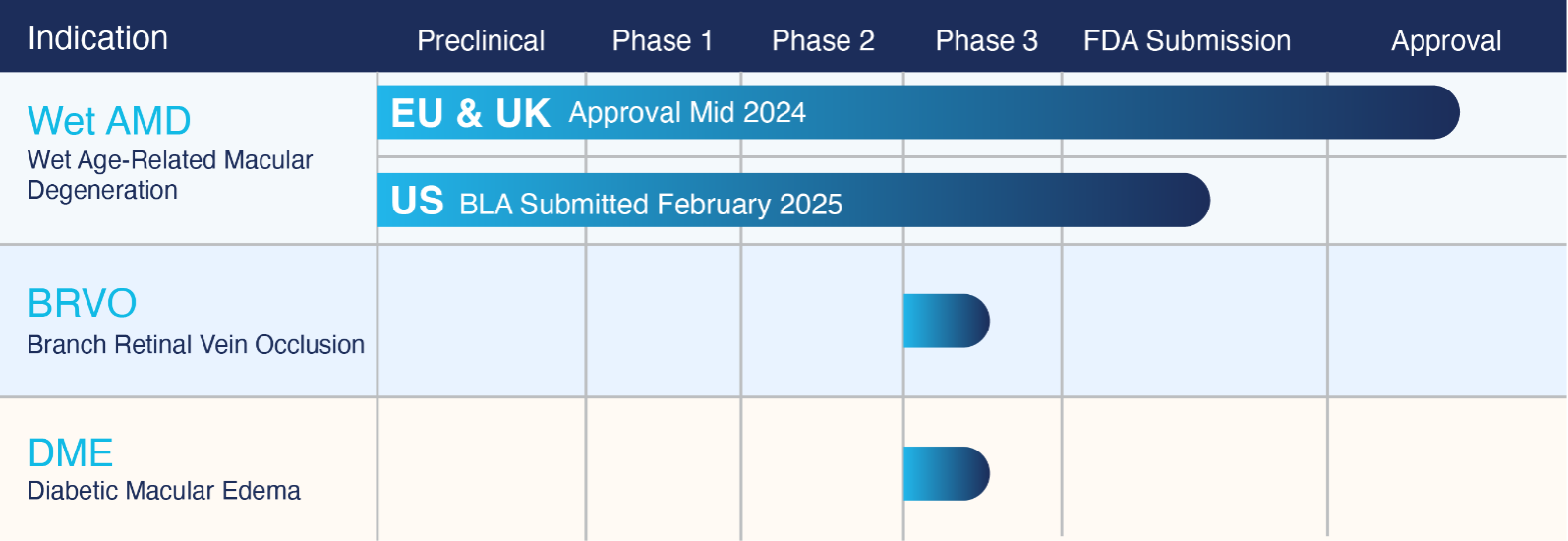
✓
ONS-5010 Received MHRA (Medicines & Healthcare Products Regulatory Agency) Authorization as LYTENAVA™ (bevacizumab gamma) for the Treatment of Wet AMD in July 2024
Trusted Medicine. Proven Progress.
We are bringing ophthalmic-grade precision to bevacizumab. Our manufacturing process focuses on consistent quality and our therapy is supported by comprehensive clinical trials designed to validate safety and efficacy, building the trusted evidence physicians need for confident treatment decisions.

