
ONS-5010 / LYTENAVA™ (bevacizumab-vikg) — Investigational Ophthalmic Formulation of Bevacizumab
ONS-5010 / LYTENAVA™ (bevacizumab-vikg) is an investigational ophthalmic formulation of bevacizumab under development to be administered as an intravitreal injection for the treatment of wet AMD and other approved retinal diseases. Since no currently approved ophthalmic formulations of bevacizumab are available, clinicians wishing to treat retinal patients with bevacizumab have had to use unapproved repackaged IV bevacizumab provided by compounding pharmacies, products that have risk of contamination and inconsistent potency and availability. If approved, ONS-5010 will replace the need to use unapproved repackaged IV bevacizumab from compounding pharmacies for the treatment of wet AMD. We currently intend to commercialize ONS-5010 in both vial and pre-filled syringe formulations.
Bevacizumab-vikg is a recombinant humanized monoclonal antibody (mAb) that selectively binds with high affinity to all isoforms of human vascular endothelial growth factor (VEGF) and neutralizes VEGF’s biologic activity through a steric blocking of the binding of VEGF to its receptors Flt-1 (VEGFR-1) and KDR (VEGFR-2) on the surface of endothelial cells. Following intravitreal injection the binding of bevacizumab-vikg to VEGF prevents the interaction of VEGF with its receptors on the surface of endothelial cells, reducing endothelial cell proliferation, vascular leakage, and new blood vessel formation in the retina.
Since the advent of anti-VEGF therapy, it has become the standard-of-care treatment option within the retina community globally (National Eye Institute).
About AMD and Anti-VEGF Therapy

Wet AMD is the leading cause of vision loss among people age 50 and older*

34.5 million patient prevalence for retinal diseases (wet AMD, diabetic retinopathy/diabetic macular edema and retinal vein occlusion) in the United States, United Kingdom, EU, China, Japan and Australia**

Anti-VEGF therapy is the global standard of care for wet AMD within the retina community

Estimated annual 2020 revenue is $13.1 billion in the nine major markets from FDA-approved anti-VEGFs for ophthalmic use**
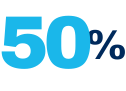
Use of unapproved repackaged IV bevacizumab from compounding pharmacies estimated to account for at least 50% of all wet AMD prescriptions in the United States**
What is AMD?
Age-related macular degeneration, AMD, is a common eye condition and a leading cause of vision loss among people age 50 and older. It causes damage to the macula, a small spot near the center of the retina and the part of the eye needed for sharp, central vision, which lets us see objects that are straight ahead.*
What is "Wet" AMD?
Wet AMD is a form of “late stage” AMD, and is also called neovascular AMD. In wet AMD, abnormal blood vessels grow underneath the retina. These vessels can leak fluid and blood, which may lead to swelling and damage of the macula, causing vision loss.*
What is VEGF?
With wet AMD, abnormally high levels of vascular endothelial growth factor (VEGF) are secreted in the eyes. VEGF is a protein that promotes the growth of new abnormal blood vessels. Anti-VEGF injection therapy blocks this growth.*
How Established is Anti-VEGF Therapy?
Since the advent of anti-VEGF therapy, it has become the standard-of-care treatment option within the retina community globally.
Retinal diseases, including wet AMD, diabetic retinopathy/diabetic macular edema, and retinal vein occlusion, are significant diseases worldwide, with a prevalence of 34.5 million patients in the United States, United Kingdom, top four European countries, Japan, China and Australia in 2020. In 2020, total revenue from FDA-approved anti-VEGFs is estimated to exceed $13.1 billion annually in those nine ophthalmic markets.**
Although not currently FDA-approved for use in treating wet AMD, unapproved repackaged intravenous (IV) bevacizumab from compounding pharmacies is believed to account for approximately 50% of all wet AMD prescriptions in the United States.
